This scientific and business case evaluation was completed as part of the Entrepreneurship in Healthcare and Life Science course at Chicago Booth taught by serial entrepreneur Brian Coe.
The company evaluation deck can be found here.
October 2025
Kyverna Therapeutics
Scientific and Business Case Evaluation
Entrepreneurship in Healthcare & Life Science – Midterm
Overview
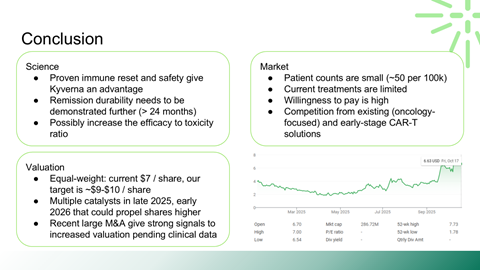
This project is a fundamental evaluation of Kyverna Therapeutics, a publicly traded biotechnology company developing CAR-T cell therapies for severe autoimmune diseases. The work mirrors a public equity research process rather than a venture pitch.
Analytical Focus
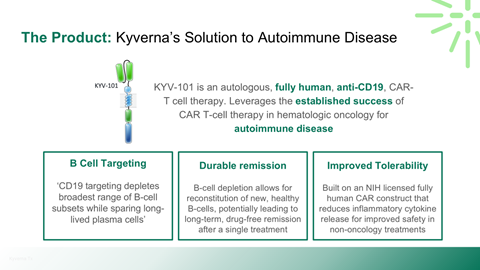
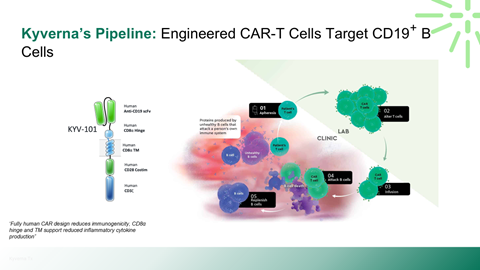
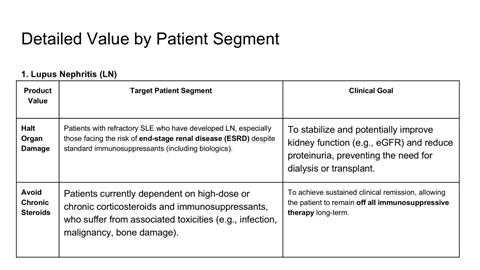
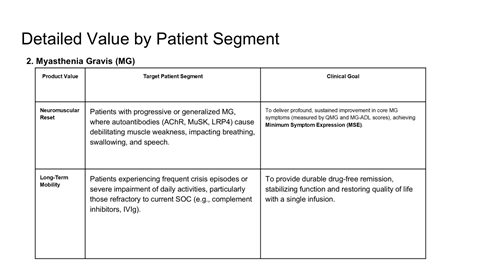
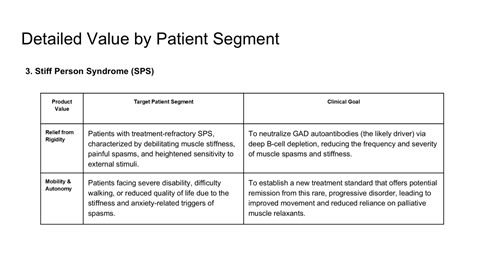
Scientific rationale for applying CAR-T therapy beyond oncology
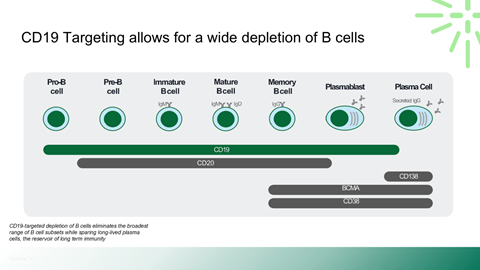
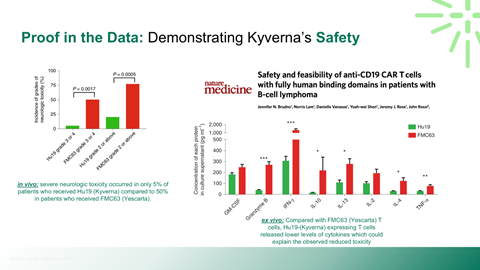
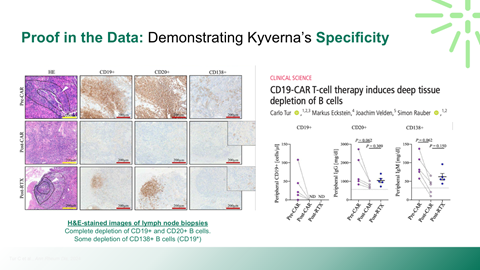
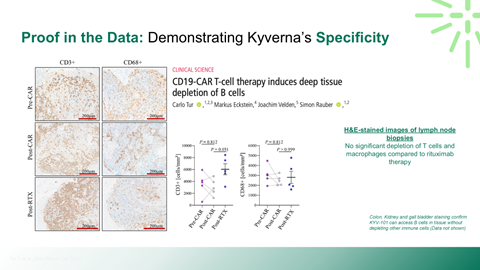
Safety, durability, and relapse risk associated with deep B-cell depletion
Manufacturing scalability as a commercial constraint
Valuation sensitivity to upcoming clinical and regulatory catalysts
Scope of Analysis
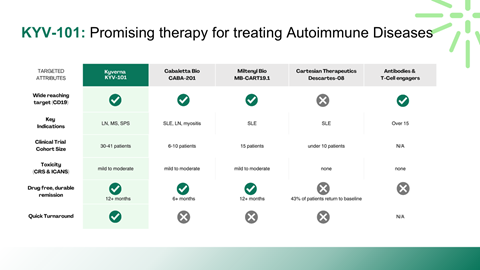
Mechanism of action and differentiation versus existing biologics
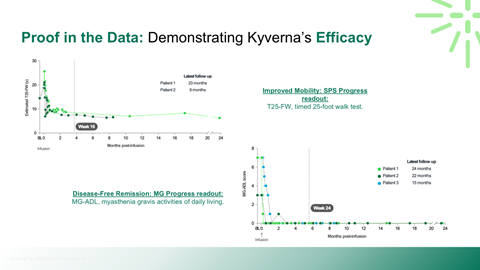
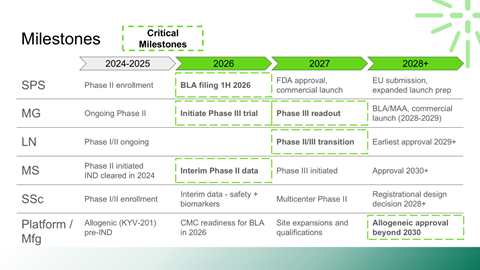
Interpretation of Phase II clinical data and transition risks to Phase III
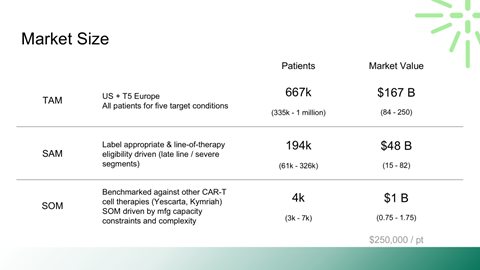
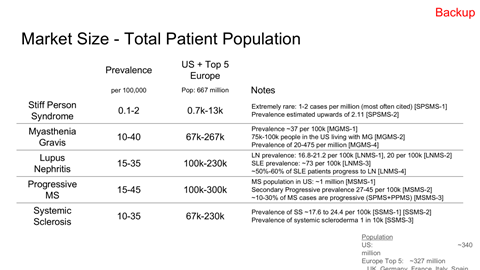
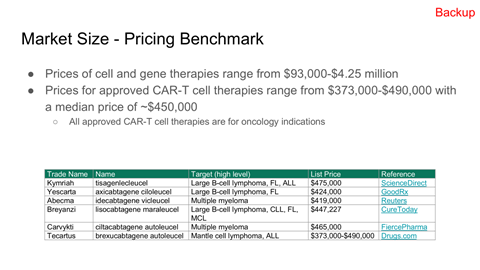
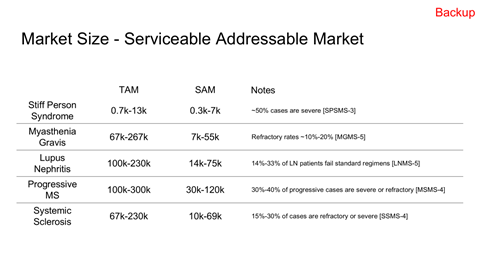
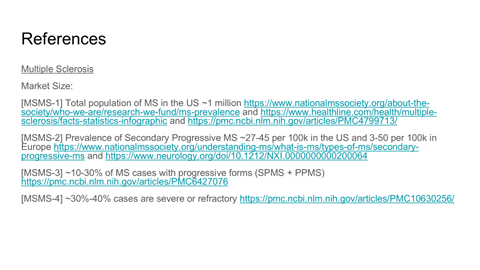
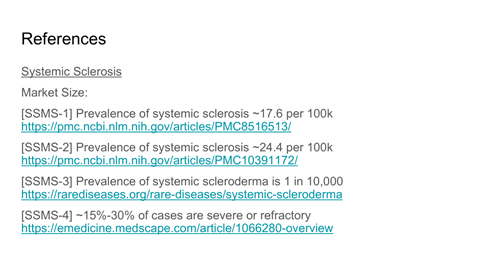
TAM, SAM, and capacity-constrained SOM modeling
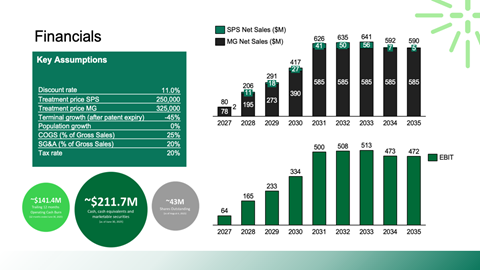
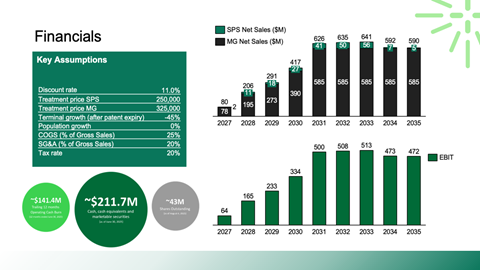
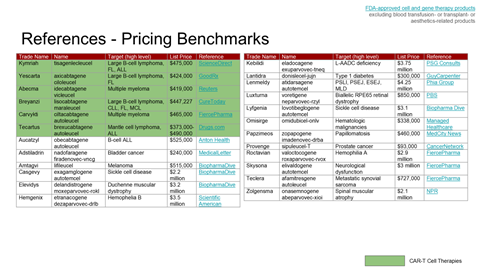
Valuation framing using public comparables and precedent transactions
Why This Work Matters
Advanced cell therapies often attract enthusiasm that outpaces evidence. This analysis demonstrates a disciplined approach to evaluating cutting-edge science while grounding conclusions in clinical data, manufacturing realities, and capital markets behavior.
My Role
Led business case evaluation
Built market sizing and capacity-constrained adoption assumptions
Contributed to valuation framing and investment conclusion
Synthesized findings into an scientific and business case assessment